Draw The Lewis Structure Of Ammonia Nh3
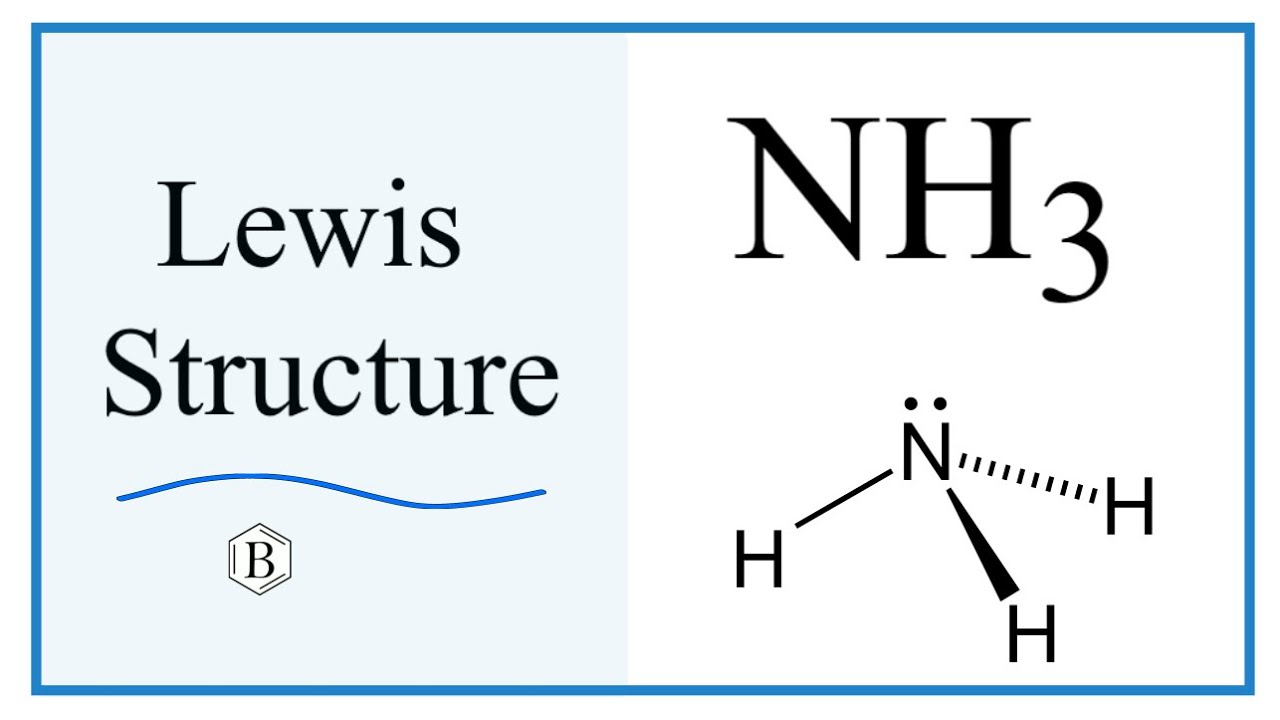
Draw The Lewis Structure Of Ammonia Nh3 - Web drawing the lewis structure for nh 3. Here, the given molecule is nh3 (ammonia). Web the molar mass of the ammonia molecule is 17.031 g/mol. Web this video outlines how to draw the lewis structure for ammonia, or nh3 #chem #chemistry #generalchemistry #lewisstructure #science #polarity #polar. The lewis structure of a molecule helps understand the electron geometry, molecular. This will help you understand the molecule’s electronic structure. Web to draw the nh3 lewis structure (ammonia) involves a few straightforward steps. Understand the bonding and electron distribution in ammonia. Web the lewis structure of ammonia, #nh_3#, would be three hydrogen atoms bonded to a nitrogen atom in the middle, with a lone pair of electrons on top of the. Web steps to draw the lewis structure of nh 3. Understand the bonding and electron distribution in ammonia. Web to illustrate this method, let’s calculate the formal charge on the atoms in ammonia (nh3) whose lewis electron structure is as follows: Web craig beals shows how to draw the lewis structure for ammonia.this is a clip from the complete video: Web steps to draw the lewis structure of nh 3. Web drawing the lewis structure for nh 3. In order to find the total valence electrons in nh3 molecule,. Note that hydrogen is often. Web to draw the lewis structure of nh3, you need to determine the total number of valence electrons in the molecule. Web steps of drawing nh3 lewis structure step 1: Web the lewis structure of ammonia, #nh_3#, would be three hydrogen atoms bonded to a nitrogen atom in the middle, with a lone pair of electrons on top of the. Web steps to draw the lewis structure of nh 3. Find the total valence electrons in nh3 molecule. This will help you understand the molecule’s electronic structure. Nh 3 is made up of two atoms and both have a total of eight electrons in the. Web drawing the lewis structure for nh 3. Web this video outlines how to draw the lewis structure for ammonia, or nh3 #chem #chemistry #generalchemistry #lewisstructure #science #polarity #polar. Find the total valence electrons in nh3 molecule. Web the molar mass of the ammonia molecule is 17.031 g/mol. Calculate the total number of valence electrons. Web the lewis structure of ammonia, #nh_3#, would be three hydrogen atoms bonded. Web to draw the lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. Web drawing the lewis structure for nh 3. Web steps of drawing nh3 lewis structure step 1: Note that hydrogen is often. Web the lewis structure of ammonia, #nh_3#, would be three hydrogen atoms bonded. Web to draw the nh3 lewis structure (ammonia) involves a few straightforward steps. The melting point and boiling point of the ammonia molecule are 195.42 k and 239.81 k. Web the molar mass of the ammonia molecule is 17.031 g/mol. Web drawing the nh3 lewis structure involves following a set of rules and guidelines. Calculate the total number of electrons. Web there is really only one way to draw the lewis structure for ammonia (nh3). Find the total valence electrons in nh3 molecule. Web to draw the lewis structure of nh3, you need to determine the total number of valence electrons in the molecule. A neutral nitrogen atom has five valence. Valence electrons are the electrons. Web drawing the nh3 lewis structure involves following a set of rules and guidelines. Web to draw the lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. Calculate the total number of electrons. Web ammonia (nh 3) is a commonly tested lewis structure due to it's widespread use. Web the lewis structure of ammonia, #nh_3#, would be three hydrogen atoms bonded to a nitrogen atom in the middle, with a lone pair of electrons on top of the. Web ammonia or nh3 has a total of 8 valence electrons. Understand the bonding and electron distribution in ammonia. Note that hydrogen is often. The lewis structure of a molecule. The lewis structure of a molecule helps understand the electron geometry, molecular. Understand the bonding and electron distribution in ammonia. Web 6 steps to draw the lewis structure of nh3 step #1: Web this video outlines how to draw the lewis structure for ammonia, or nh3 #chem #chemistry #generalchemistry #lewisstructure #science #polarity #polar. Here, the given molecule is nh3 (ammonia). The melting point and boiling point of the ammonia molecule are 195.42 k and 239.81 k. Web steps of drawing nh3 lewis structure step 1: Find the total valence electrons in nh3 molecule. Web drawing the nh3 lewis structure involves following a set of rules and guidelines. Web a video explanation of how to draw the lewis dot structure for. Web understanding the nh3 lewis structure is crucial for comprehending the chemical properties and behavior of ammonia. Web drawing the lewis structure for nh 3. Web steps of drawing nh3 lewis structure step 1: Web the lewis structure of ammonia, #nh_3#, would be three hydrogen atoms bonded to a nitrogen atom in the middle, with a lone pair of electrons. Web the lewis structure of ammonia, #nh_3#, would be three hydrogen atoms bonded to a nitrogen atom in the middle, with a lone pair of electrons on top of the. The melting point and boiling point of the ammonia molecule are 195.42 k and 239.81 k. It's not particularly difficult but is an important structure. Web craig beals shows how to draw the lewis structure for ammonia.this is a clip from the complete video: Nh 3 (ammonia) is a commonly tested lewis structure. Web a video explanation of how to draw the lewis dot structure for ammonia, along with information about the compound including formal charges, polarity, hybrid. In order to find the total valence electrons in nh3 molecule,. For resonance structures there must be a double or triple bond present, which is. Web to draw the lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. Web understanding the nh3 lewis structure is crucial for comprehending the chemical properties and behavior of ammonia. Valence electrons are the electrons. Web to illustrate this method, let’s calculate the formal charge on the atoms in ammonia (nh3) whose lewis electron structure is as follows: Understand the bonding and electron distribution in ammonia. Calculate the total number of electrons. Web steps to draw the lewis structure of nh 3. Web ammonia or nh3 has a total of 8 valence electrons.Nh3 Molecule Structure
NH3 Lewis Structure (Ammonia) YouTube
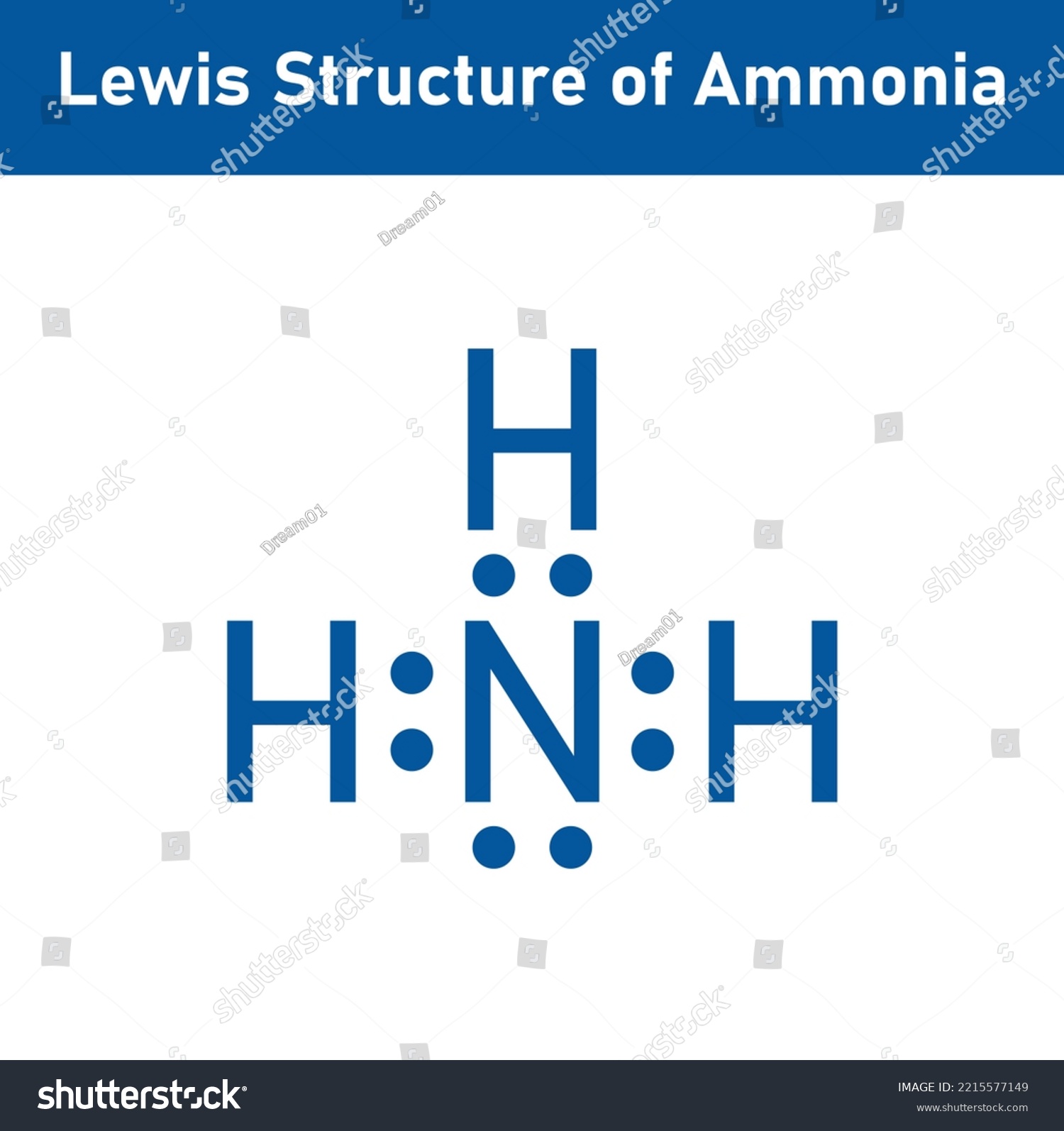
Lewis Structure Ammonia Nh3 Scientific Vector Stock Vector (Royalty
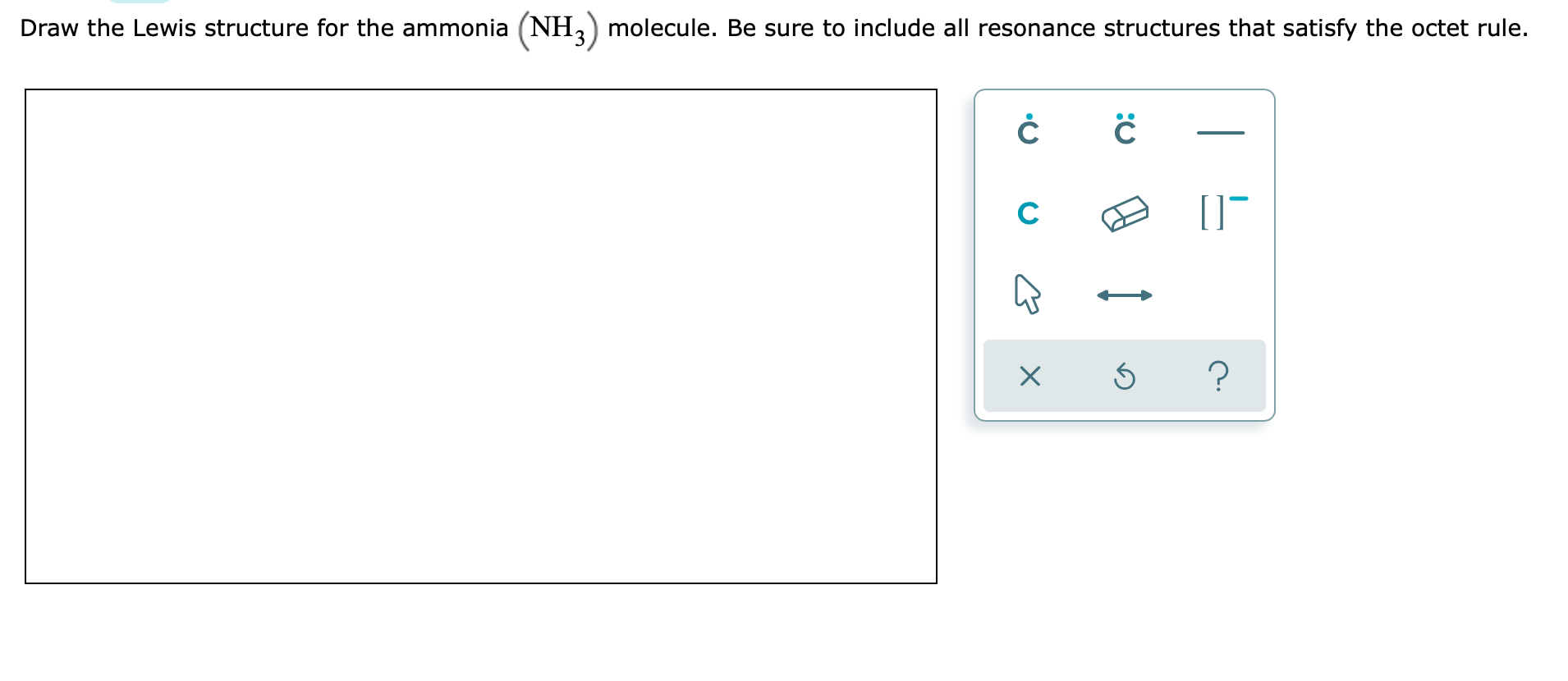
Solved Draw the Lewis structure for the ammonia (NH3)
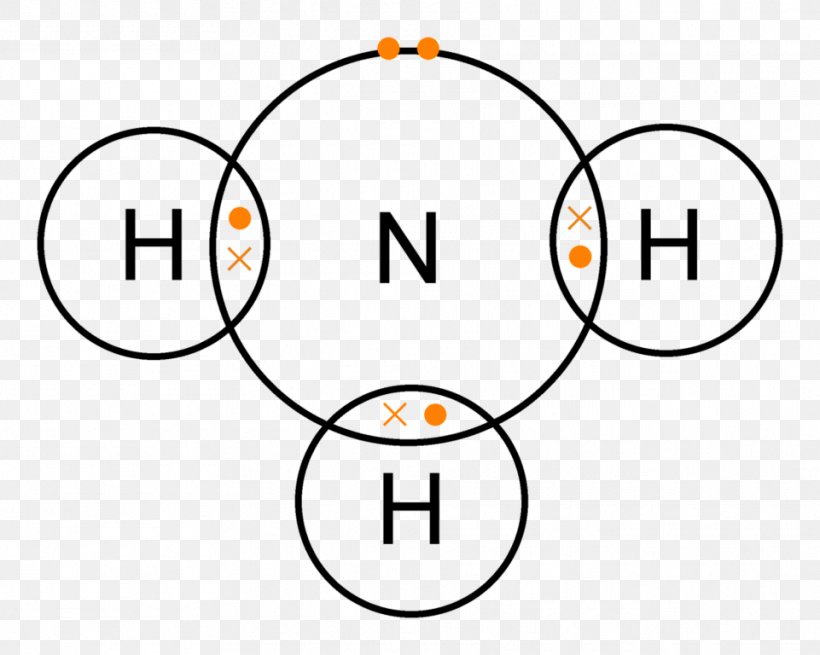
Lewis Dot Structure Of Nh3
Molecular Structure of Ammonia (NH3) YouTube
NH3 Molecular Geometry / Shape and Bond Angles (Ammonia) YouTube
Draw the Lewis structure for the ammonia (NH3)
NH3 Lewis Structure How to Draw the Dot Structure for NH3 (Ammonia
Lewis Structure Ammonia Covalent Bond Lone Pair Chemical Bond, PNG
Web This Chemistry Video Tutorial Explains How To Draw The Lewis Structure Of Nh3 Also Known As Ammonia.how To Draw Lewis Structures:
Web 6 Steps To Draw The Lewis Structure Of Nh3 Step #1:
Web The Molar Mass Of The Ammonia Molecule Is 17.031 G/Mol.
Web Ammonia (Nh 3) Is A Commonly Tested Lewis Structure Due To It's Widespread Use In Agriculture As A Fertilizer.it Also Is A Good Example Of A Molecule With A Trigonal Prymidal.
Related Post: