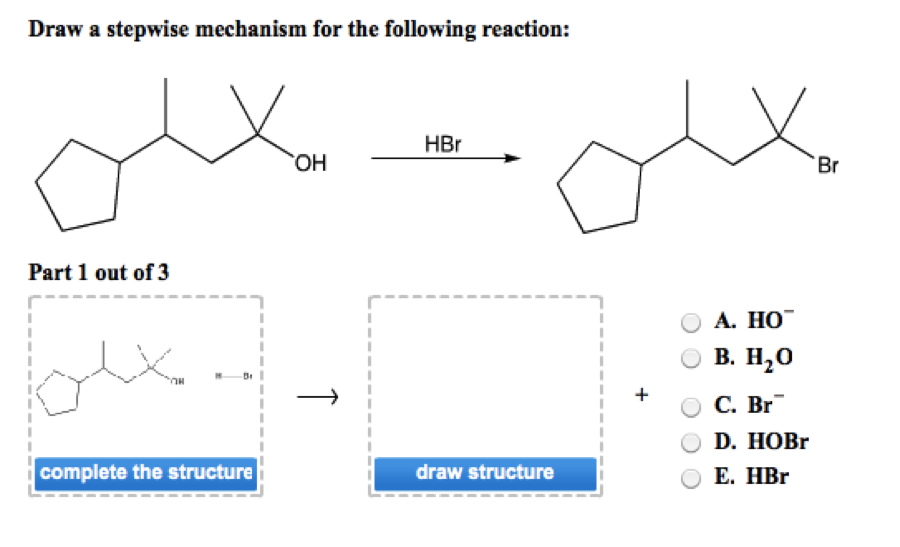
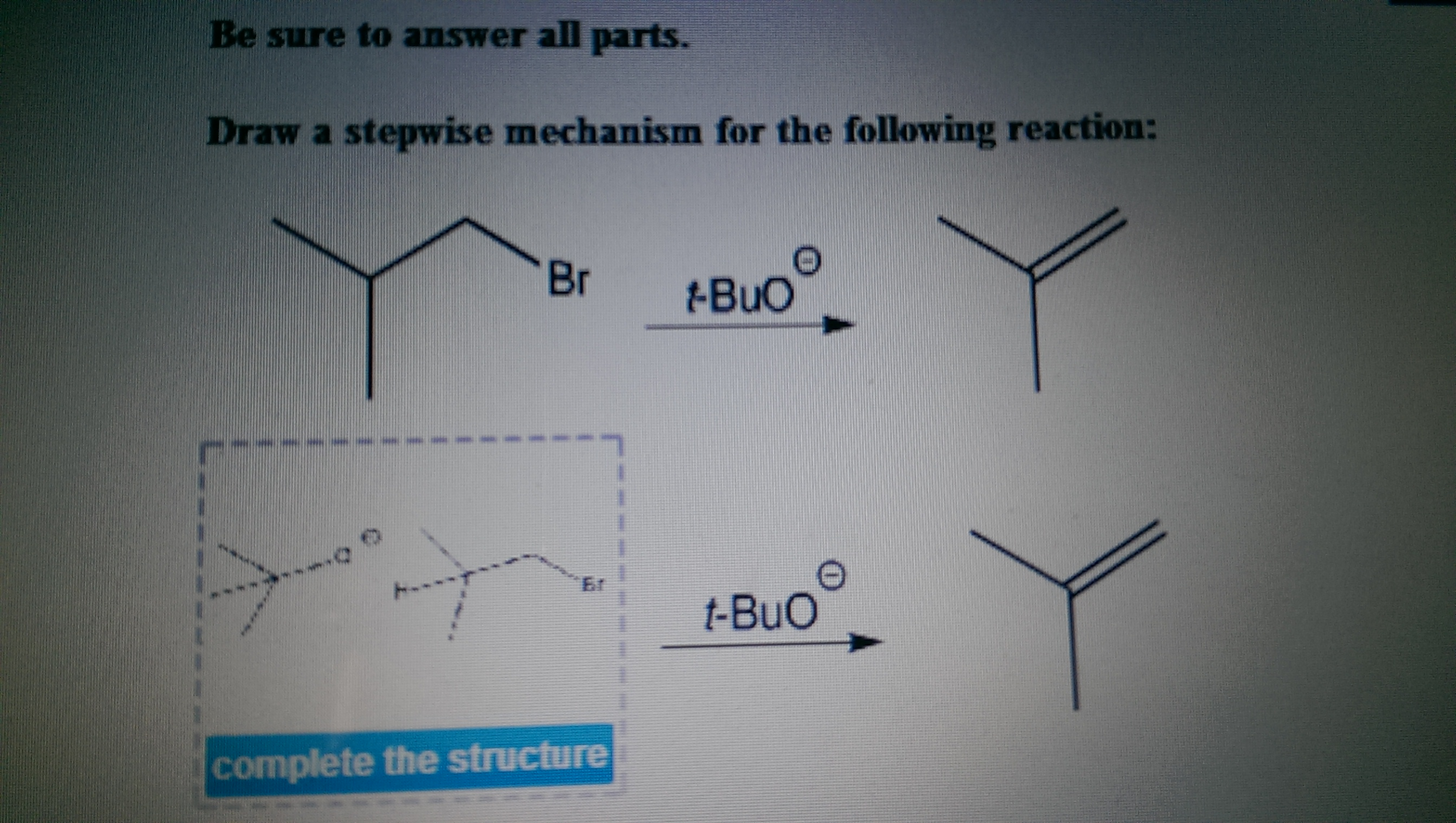
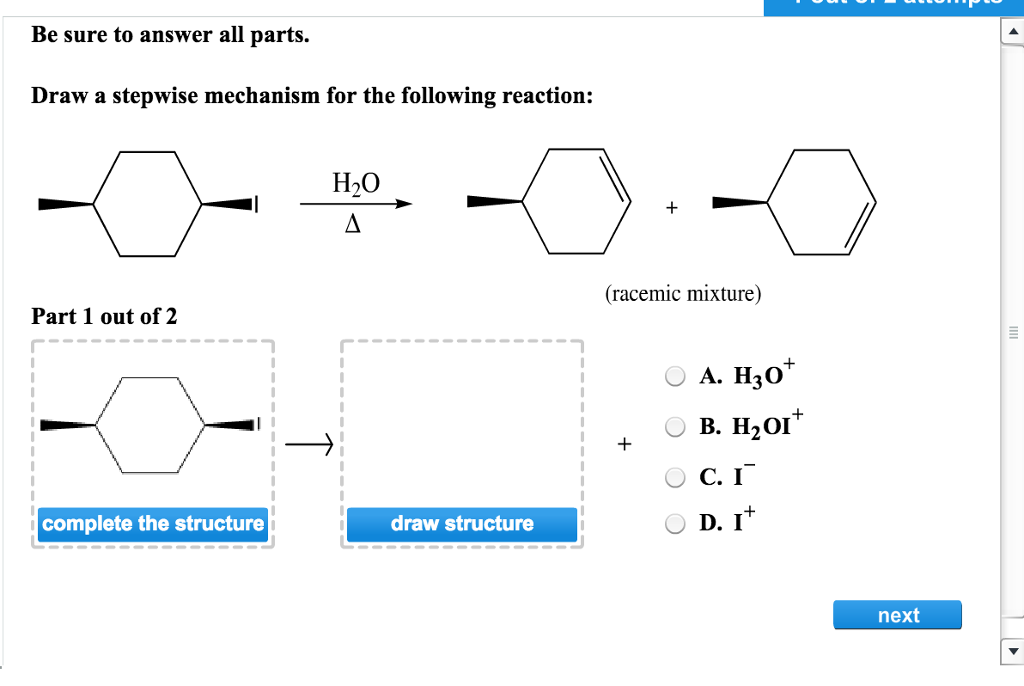
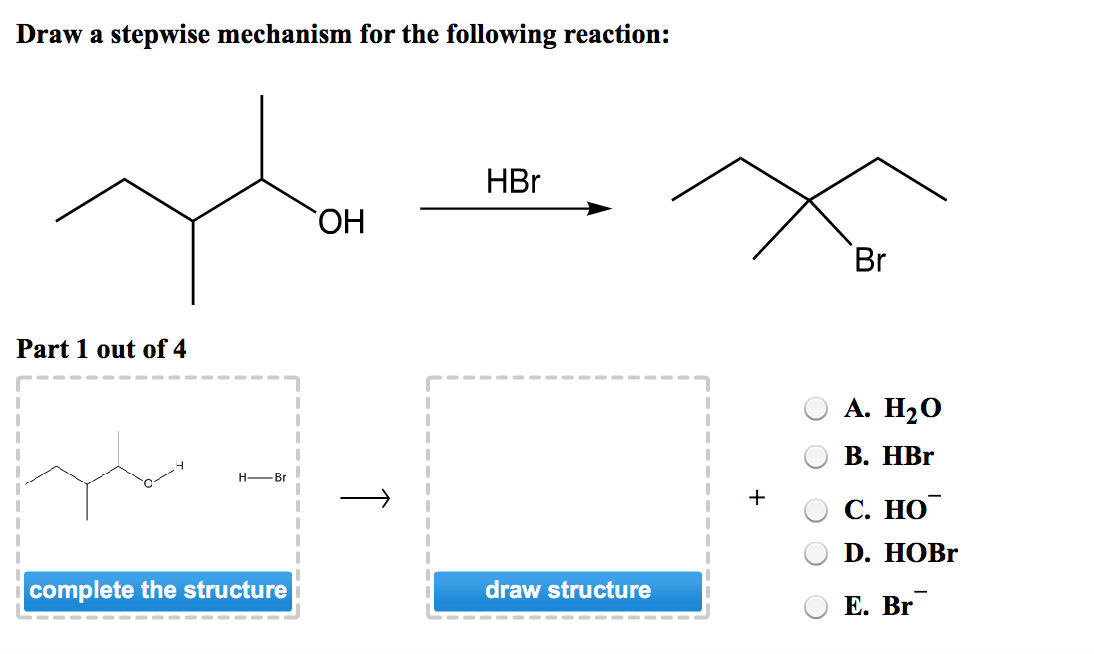
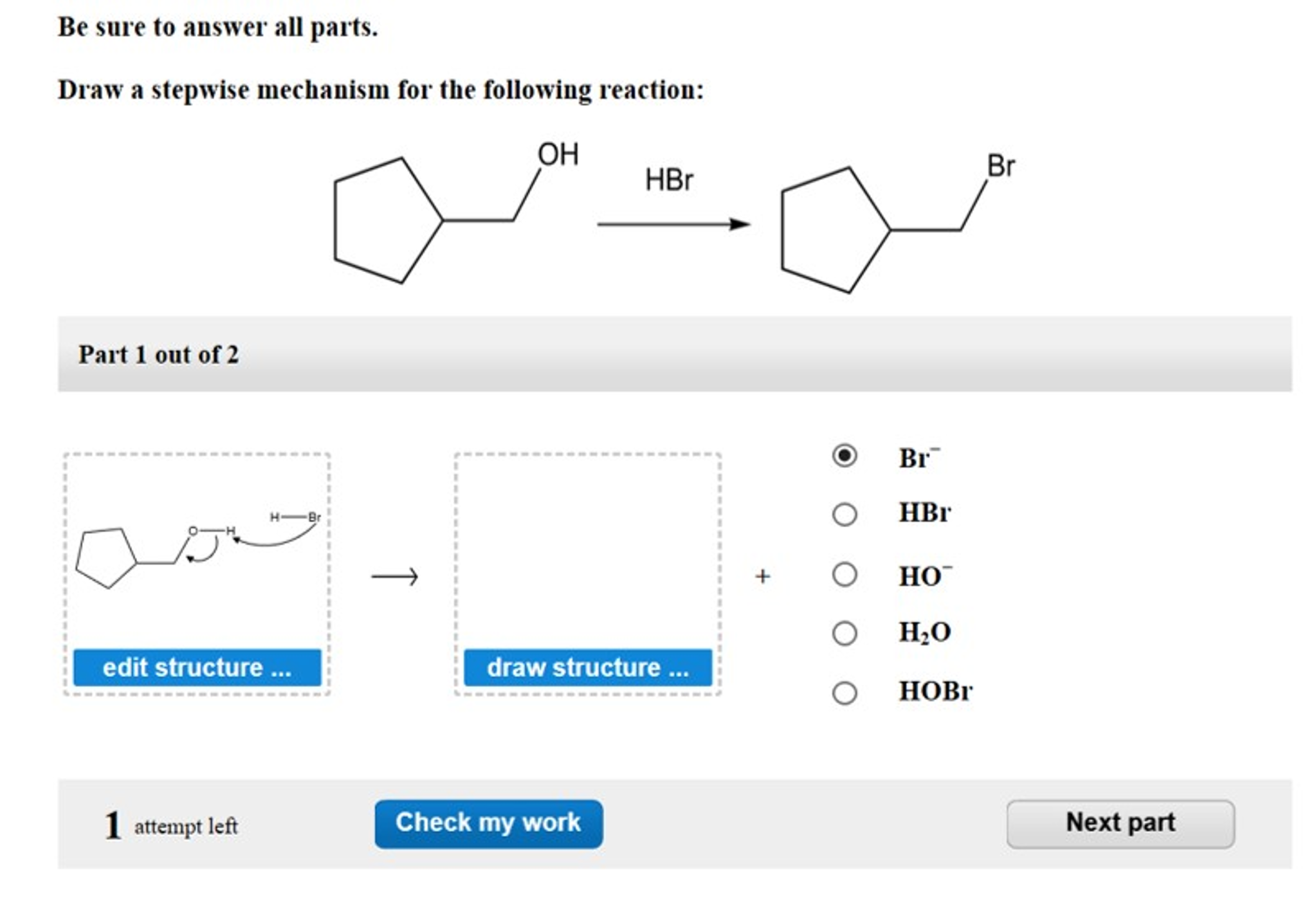
Draw A Stepwise Mechanism For The Following Reaction
Draw A Stepwise Mechanism For The Following Reaction - A reaction intermediate is a chemical species that is formed in one elementary step and consumed in a subsequent step. There are 3 steps to solve this one. There are 2 steps to solve this one. Web define an elementary reaction, and state how it differs from an ordinary net chemical reaction. Here’s the best way to solve it. O 3(g) o 2(g) + oo + o 3(g) 2o 2(g) we call each step in a reaction mechanism an elementary reaction. Web the first step for the given reaction involves the addition of halogen to the acetylene molecule and results in the formation of a cyclic intermediate. Web draw a stepwise mechanism for the following reaction: 100% (4 ratings) share share. The decomposition of ozone, for example, appears to follow a mechanism with two steps: Web draw a stepwise mechanism for the following reaction: Web draw a stepwise mechanism for the following reaction: O 3(g) o 2(g) + oo + o 3(g) 2o 2(g) we call each step in a reaction mechanism an elementary reaction. A chemical reaction often occurs in steps, although it may not always be obvious to an observer. Web a balanced equation indicates what is reacting and what is produced, but it reveals no details about how the reaction actually takes place. 100% (8 ratings) share share. Here’s the best way to solve it. Be sure to answer all parts. The water molecule then acts as a nucleophile and leads to the formation of carbocation intermediate, which is. I need help on part two. Be sure to answer all parts draw a stepwise mechanism for the following reaction: View the full answer step 2. 100% (1 rating) share share. A mechanism refers to the series of steps that the reagents undergo during a chemical reaction. Web the reaction mechanism (or reaction path) is the process, or pathway, by which a reaction occurs. Web draw a stepwise mechanism for the following reaction: Web write a balanced chemical equation for a process given its reaction mechanism. A chemical reaction often occurs in steps, although it may not always be obvious to an observer. Be sure to answer all parts. Here’s the best way to solve it. The decomposition of ozone, for example, appears to follow a mechanism with two steps: Here first oxygen attack on the socl2 and chloride ion r. Oh h,so 4 part 1 out of 4 oa. A reaction intermediate is a chemical species that is formed in one elementary step and consumed in a subsequent step. Just need help on the last. Here first oxygen attack on the socl2 and chloride ion r. Web write a balanced chemical equation for a process given its reaction mechanism. Web a chemical reaction often occurs in steps, although it may not always be obvious to an observer. There are 2 steps to solve this one. Be sure to answer all parts. Be sure to answer all parts. A reaction intermediate is a chemical species that is formed in one elementary step and consumed in a subsequent step. Derive the rate law consistent with a given reaction mechanism. Oh h,so 4 part 1 out of 4 oa. Be sure to answer all parts. Web this reaction combines two processes together: Oh h,so 4 part 1 out of 4 oa. Be sure to answer all parts. View structure view structure part 3 out of 3 edit structure. Just need help on the last one. Oh h,so 4 part 1 out of 4 oa. Be sure to answer all parts. O 3(g) o 2(g) + oo + o 3(g) 2o 2(g) we call each step in a reaction mechanism an elementary reaction. 100% (8 ratings) share share. Web write a balanced chemical equation for a process given its reaction mechanism. I need help on part two. There are 2 steps to solve this one. Draw a stepwise mechanism for the following reaction: Draw a stepwise mechanism for the following reaction: View structure view structure part 3 out of 3 edit structure. Web write a balanced chemical equation for a process given its reaction mechanism. View structure view structure part 3 out of 3 edit structure. O 3(g) o 2(g) + oo + o 3(g) 2o 2(g) we call each step in a reaction mechanism an elementary reaction. Web a chemical reaction often occurs in steps, although it may not always be. In an internal combustion engine, for example, isooctane reacts with oxygen to give carbon dioxide and water: Web draw a stepwise mechanism for the following reaction: The water molecule then acts as a nucleophile and leads to the formation of carbocation intermediate, which is. Just need help on the last one. A reaction mechanism is the sequence of elementary steps. There are 3 steps to solve this one. Web what’s a reaction mechanism? Draw a stepwise mechanism for the following reaction: A reaction that occurs in two or more elementary steps is called a multistep or complex reaction. Web draw a stepwise mechanism for the following reaction: Be sure to answer all parts. There are 2 steps to solve this one. The decomposition of ozone, for example, appears to follow a mechanism with two steps: This is a markovnikov's addit. The decomposition of ozone, for example, appears to follow a mechanism with two steps: Be sure to answer all parts. A mechanism refers to the series of steps that the reagents undergo during a chemical reaction. Here’s the best way to solve it. There are 2 steps to solve this one. The water molecule then acts as a nucleophile and leads to the formation of carbocation intermediate, which is. 100% (1 rating) share share.Solved Draw a stepwise mechanism for the following reaction
Draw The Mechanism For The Following Reaction
Solved Draw A Stepwise Mechanism For The Following Reacti...
Solved Draw a stepwise mechanism for the following reaction
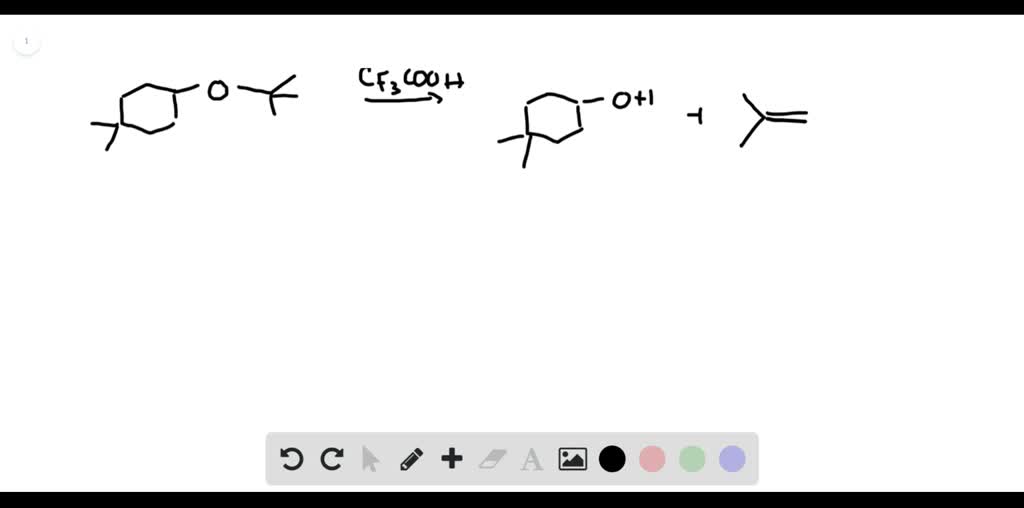
SOLVEDDraw a stepwise, detailed mechanism for the following reaction.
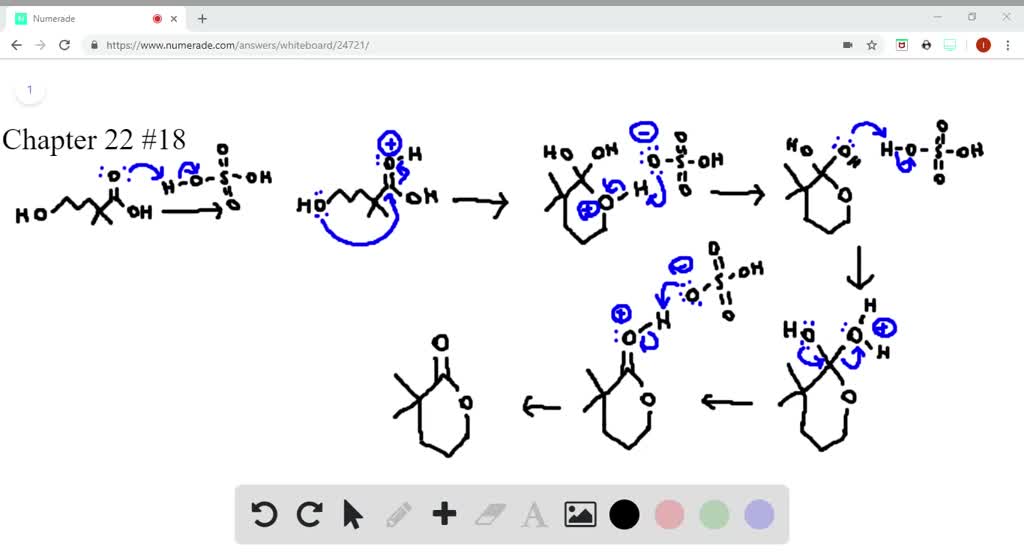
SOLVEDDraw a stepwise mechanism for the following reaction.
Solved Draw a stepwise mechanism for the following reaction
Draw A Stepwise Mechanism For The Following Reaction Hbr Sexiz Pix
OneClass VIII. Draw a stepwise reaction mechanism for the following
Draw a stepwise mechanism for the following reaction
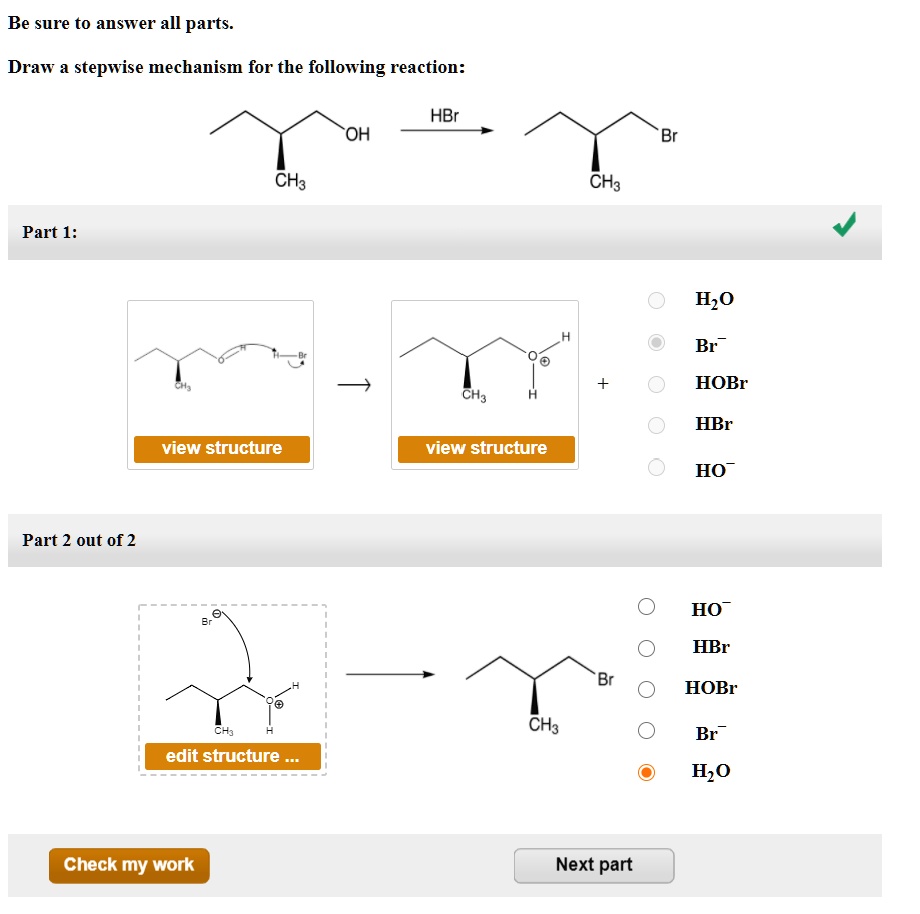
Just Need Help On The Last One.
Br H View Structure Part 2 Out Of 2 Br Edit Structure.
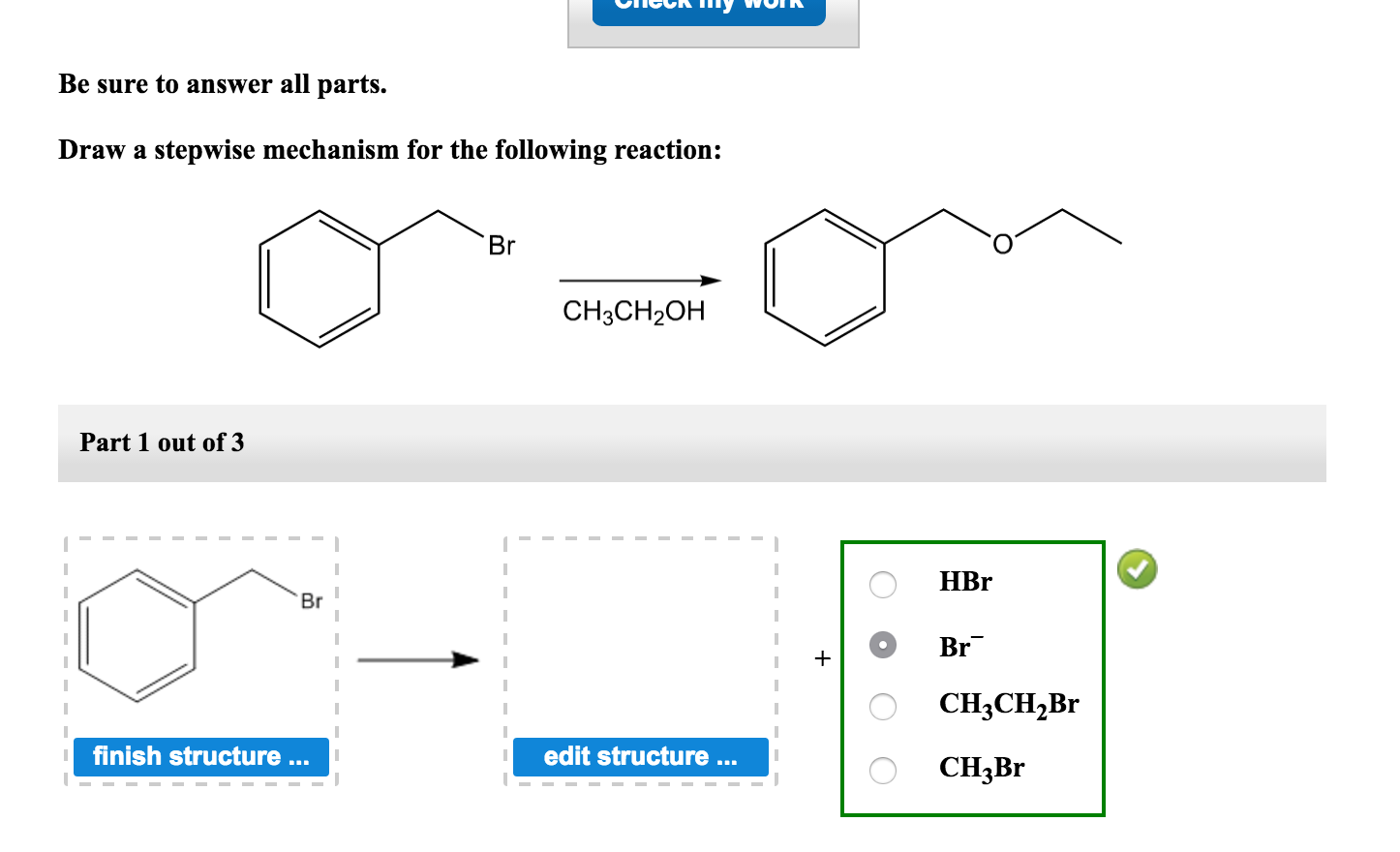
Be Sure To Answer All Parts.
View The Full Answer Step 2.
Related Post: