Bohr Model Drawing
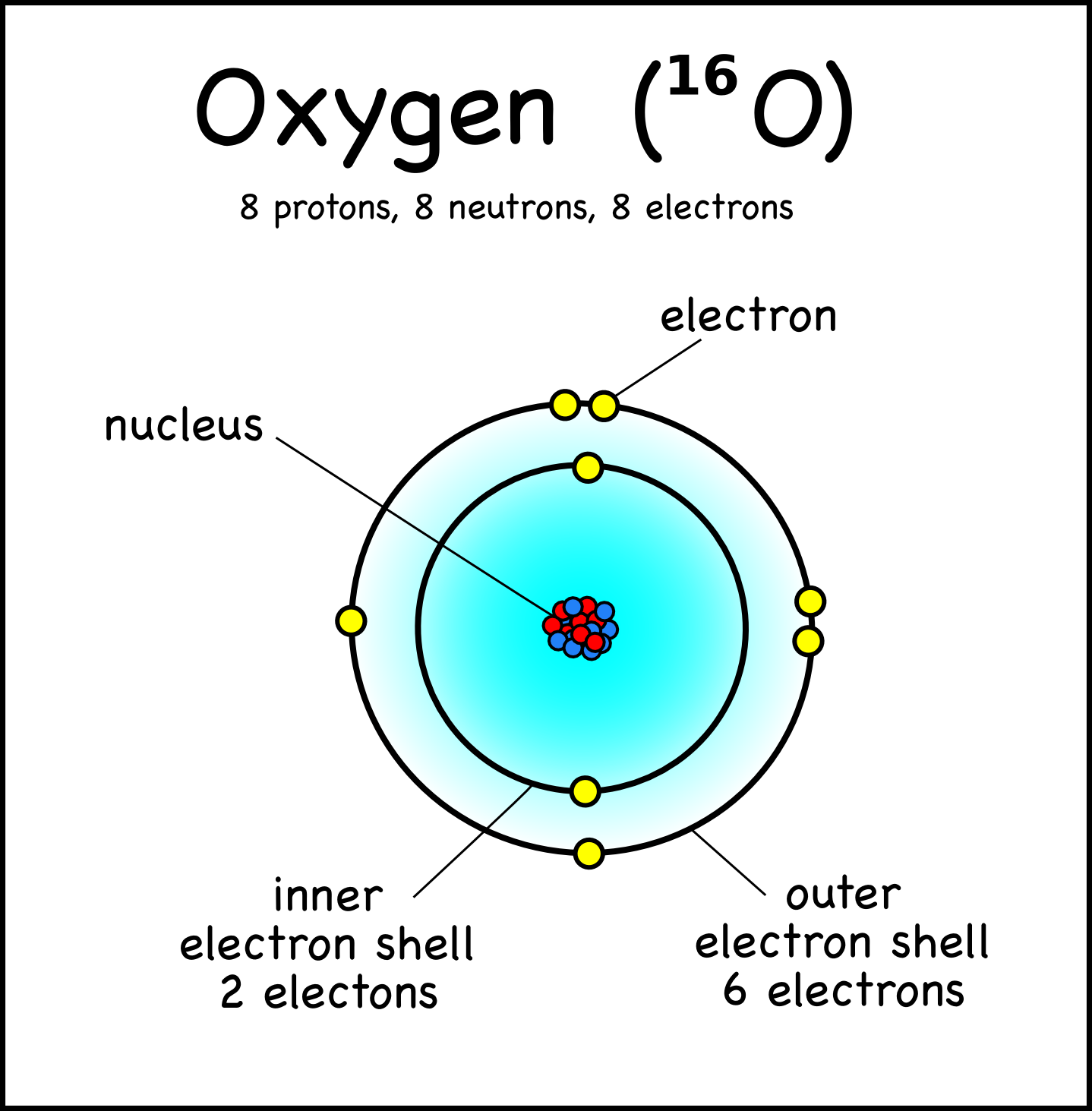
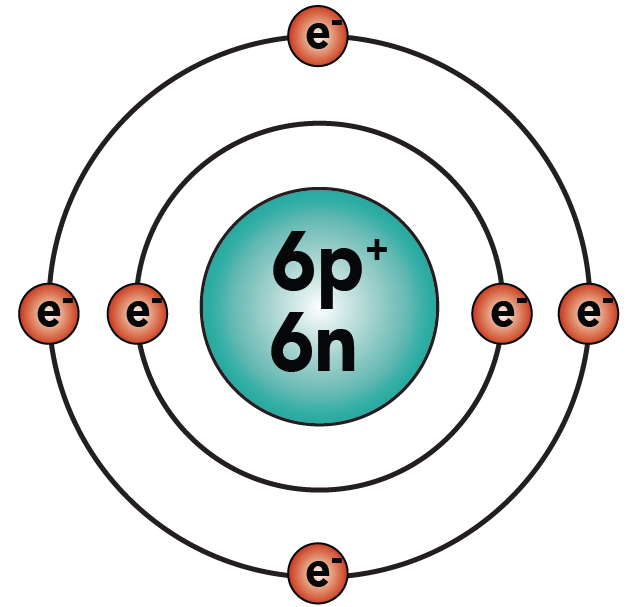
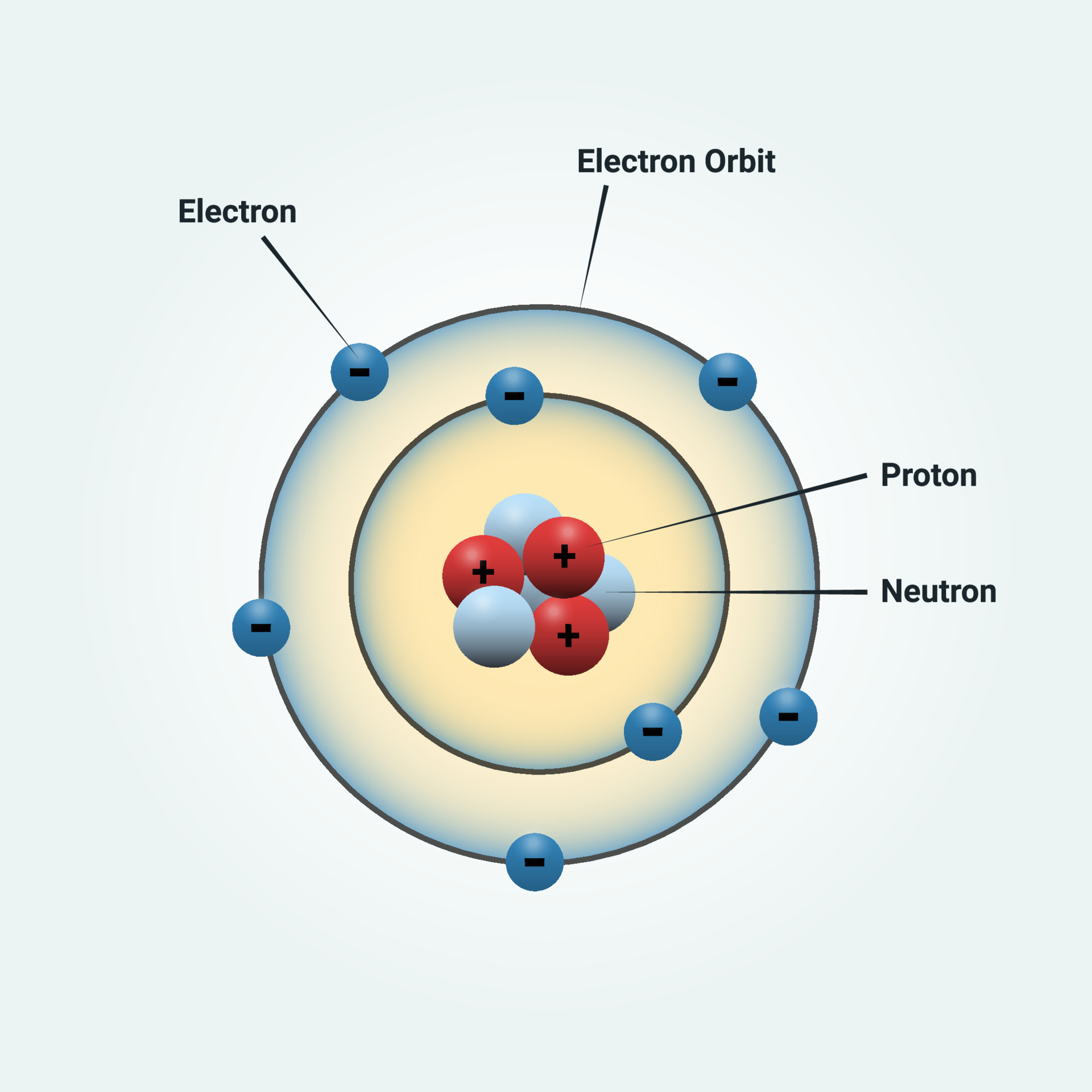
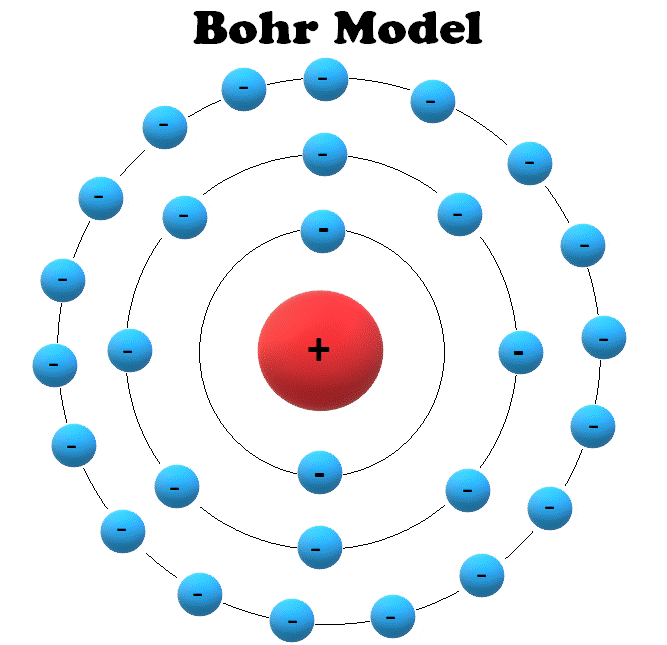
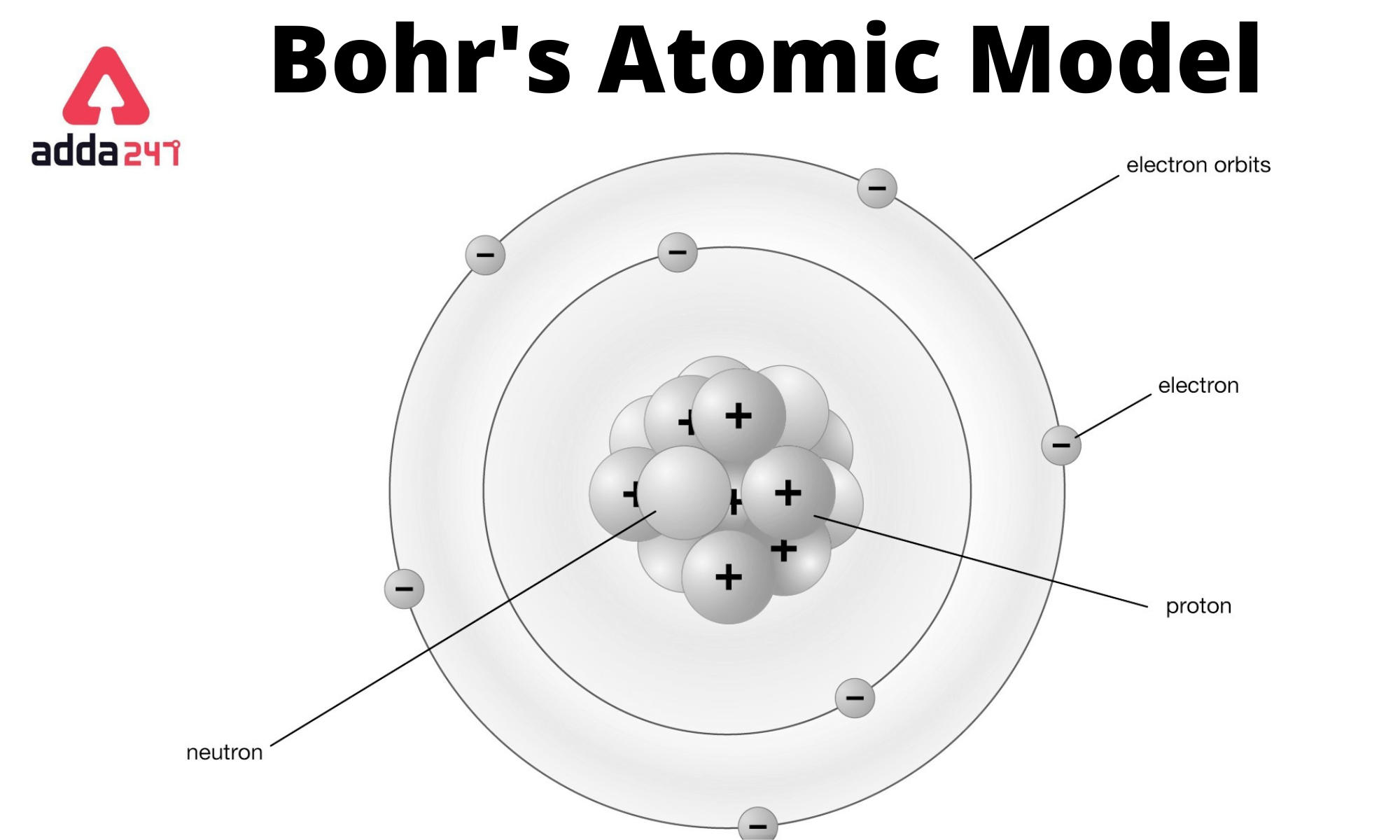
Bohr Model Drawing - You will also get the hd images of the periodic table (for free). E ( n) = − 1 n 2 ⋅ 13.6 ev. Web how did bohr respond to the drawbacks in the rutherford model? It consists of a small, dense nucleus surrounded by orbiting electrons. But what about elements with more electrons? Web helium's bohr model shows that the first two electrons are in the same energy level. Calculating electron energy for levels n=1 to 3. Web this lesson will walk your students through the basics on how to draw a bohr diagram for the first 20 elements on the periodic table. Learn how to draw a bohr model with help from an artist in this. We’ll use a bohr diagram to visually represent where the electrons are around the nucleus of the. I also created a simple worksheet for students to record their drawings and do independent practice. Bohr's model calculated the following energies for an electron in the shell, n. Web in 1913, the danish physicist niels bohr proposed a model of the electron cloud of an atom in which electrons orbit the nucleus and were able to produce atomic spectra. It consists of a small, dense nucleus surrounded by orbiting electrons. Web how did bohr respond to the drawbacks in the rutherford model? A bohr model of an oxygen atom is shown below. Get these slides and the guided notes sheets: Learn for free about math, art, computer programming, economics, physics, chemistry, biology, medicine, finance, history, and more. Calculating electron energy for levels n=1 to 3. The bohr model shows the atom as a central nucleus containing protons and neutrons with the electrons in circular orbitals at specific distances from the nucleus (figure \(\pageindex{1}\)). Web i'll teach you how to figure out the number of electron energy levels and the electrons that fit in each using the periodic table. You can effortlessly find every single detail about the elements from this single interactive periodic table. It turns out that the first energy level holds a maximum of two electrons. Web how did scientists figure. You will also learn how to write bohr model electron configuration. It consists of a small, dense nucleus surrounded by orbiting electrons. The bohr model shows the atom as a central nucleus containing protons and neutrons with the electrons in circular orbitals at specific distances from the nucleus (figure \(\pageindex{1}\)). I also created a simple worksheet for students to record. You will also learn how to write bohr model electron configuration. Understanding bohr's model requires some knowledge of electromagnetic radiation (or light). How many valence electrons does the oxygen atom have? The protons and neutrons are placed into the nucleus and the. We’ll use a bohr diagram to visually represent where the electrons are around the nucleus of the. Try out different models by shooting light at the atom. The protons and neutrons are placed into the nucleus and the. Web bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus. Get these slides and the guided notes sheets: Remember that tough the bohr model can be useful,. I also created a simple worksheet for students to record their drawings and do independent practice. You will also get the hd images of the periodic table (for free). Bohr's model calculated the following energies for an electron in the shell, n. Get these slides and the guided notes sheets: Web bohr models and lewis dot structures. Try out different models by shooting light at the atom. But what about elements with more electrons? Get these slides and the guided notes sheets: The protons and neutrons are placed into the nucleus and the. I also created a simple worksheet for students to record their drawings and do independent practice. Web helium's bohr model shows that the first two electrons are in the same energy level. Understanding bohr's model requires some knowledge of electromagnetic radiation (or light). Web i'll teach you how to figure out the number of electron energy levels and the electrons that fit in each using the periodic table. Learn for free about math, art, computer programming,. Web bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus. Web bohr model energy levels. Used to predict reactivity in elements. A bohr model of an oxygen atom is shown below. But what about elements with more electrons? Web how to draw atoms (bohr model) in this lesson i present an overview of: Used to predict reactivity in elements. Web the bohr model can be used for any atom, but the most common example you'll see is the bohr model for hydrogen. Web in this video we'll look at the atomic structure and bohr model for the oxygen. Web helium's bohr model shows that the first two electrons are in the same energy level. Web bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus. It consists of a small, dense nucleus surrounded by orbiting electrons. A bohr model of an oxygen atom is shown below. We’ll. Used to predict reactivity in elements. The bohr model shows the atom as a central nucleus containing protons and neutrons with the electrons in circular orbitals at specific distances from the nucleus (figure \(\pageindex{1}\)). Web bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus. Web i'll teach you how to figure out the number of electron energy levels and the electrons that fit in each using the periodic table. But what about elements with more electrons? Web how did scientists figure out the structure of atoms without looking at them? You will also learn how to write bohr model electron configuration. E ( n) = − 1 n 2 ⋅ 13.6 ev. Web how did bohr respond to the drawbacks in the rutherford model? Web bohr models and lewis dot structures. I also created a simple worksheet for students to record their drawings and do independent practice. Web this chemistry tutorial video walks you through how to draw a bohr model, bohr diagram, or planetary model for an atom or ion. Web in this video we'll look at the atomic structure and bohr model for the oxygen atom (o). Web the bohr model can be used for any atom, but the most common example you'll see is the bohr model for hydrogen. Web chemistry homework in 3 minutes or less!in this video, josh helps you with your chemistry homework by solving a question where we need to draw a bohr diagram. Check how the prediction of the model matches the experimental results.Potassium Bohr Model Diagram, Steps To Draw Techiescientist
Bohr Model Drawing Of Oxygen at GetDrawings Free download
Beryllium Bohr Model Diagram Wiring Diagram Pictures
Bohr atomic model of a nitrogen atom. vector illustration for science
Describe Bohrs Model of the Atom
Bohr Model of the Atom Overview and Examples
Atoms and Electrons Electronics Reference
[Solved] Bohr Model Drawing (25 points) Draw a Bohr model of an oxygen
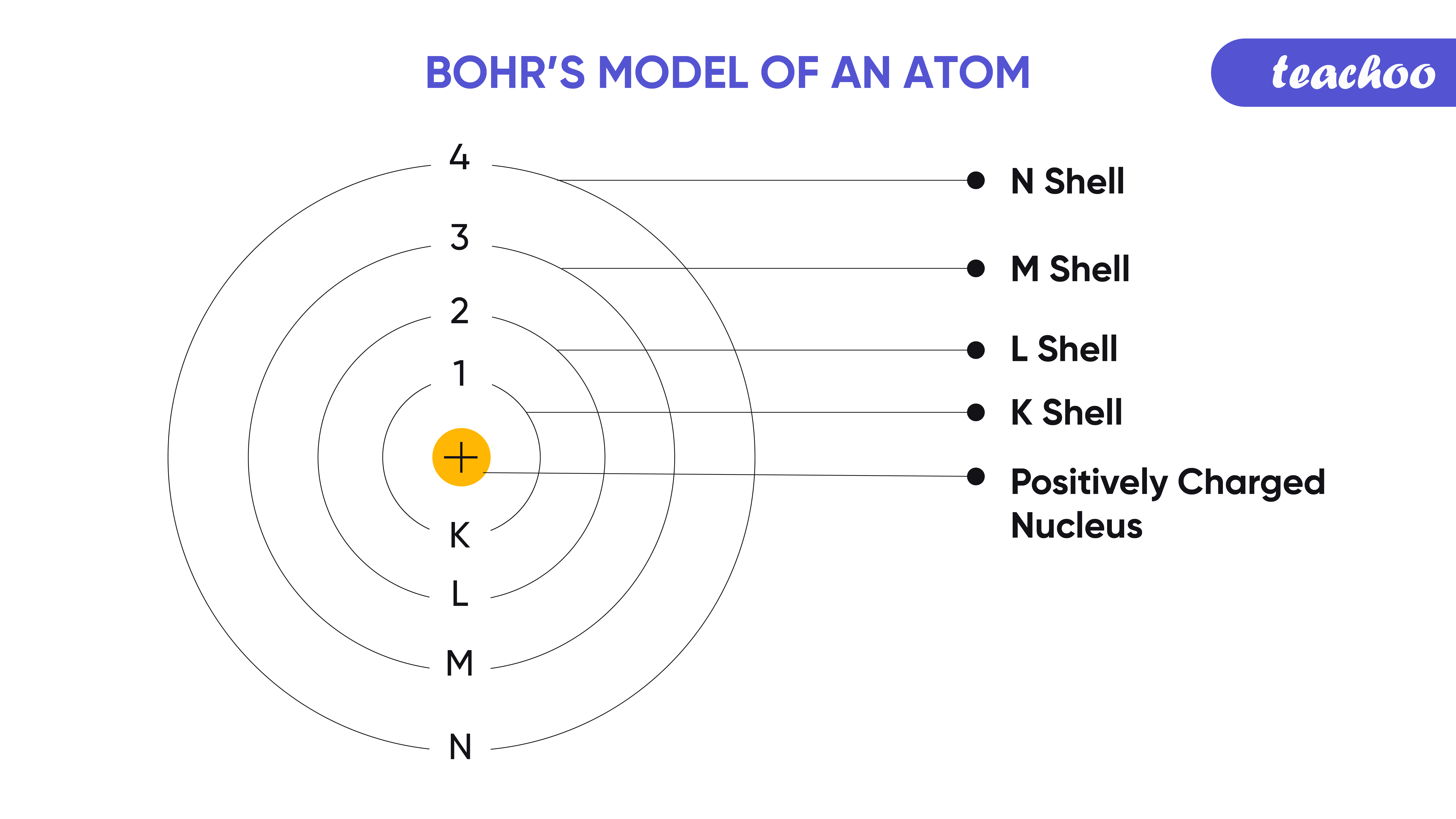
Bohr's Model of Atom Postulate and it's limitations Teachoo
Bohr Atomic Model Formula, Postulates and Limitations, Diagram
Get These Slides And The Guided Notes Sheets:
It Turns Out That The First Energy Level Holds A Maximum Of Two Electrons.
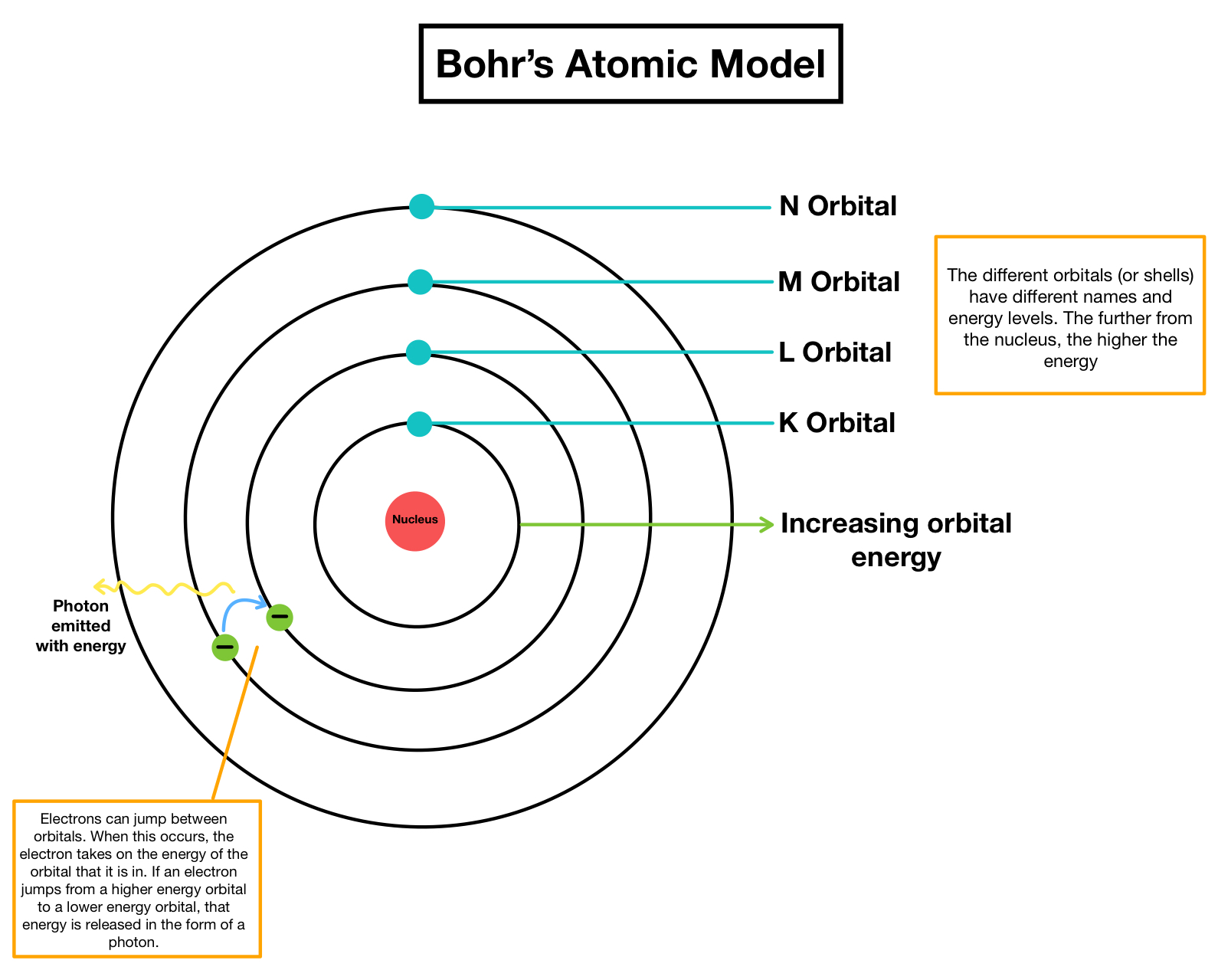
Web Bohr Model Energy Levels.
Web This Video Will Show You How To Draw Bohr Model Of Atom (Or Sometimes Known As Bohr Diagram) Correctly With Examples.
Related Post: